-
1 What is HEV (Hepatitis E Virus)?
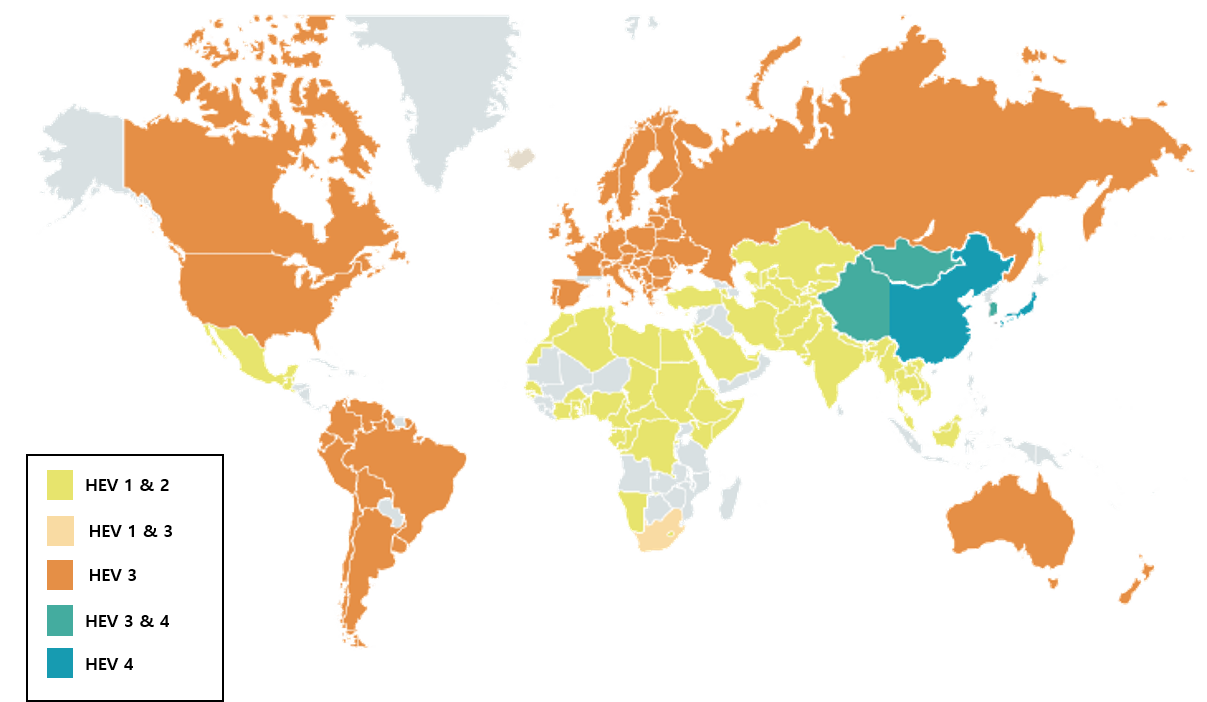
Hepatitis E Virus (HEV) is a non-enveloped, single-stranded RNA virus. HEV genotype 3 (HEV-3) primarily infects pigs and is a zoonotic virus capable of transmitting to humans.
In pigs, HEV infection can cause symptoms such as poor growth and reduced appetite. In humans, it may lead to jaundice, loss of appetite, and diarrhea. Most notably, HEV infection poses a significant risk to pregnant women, potentially causing fatal acute liver failure.
-
2 Current Status of HEV-3 Vaccines
HEV is a zoonotic virus that can infect both animals and humans, with studies showing a positivity rate of 11.5% among domestic pigs in Korea.
Currently, there are no commercially available vaccines for HEV-3, underscoring the urgent need for vaccine development to prevent zoonotic transmission and protect both animal and public health.
-
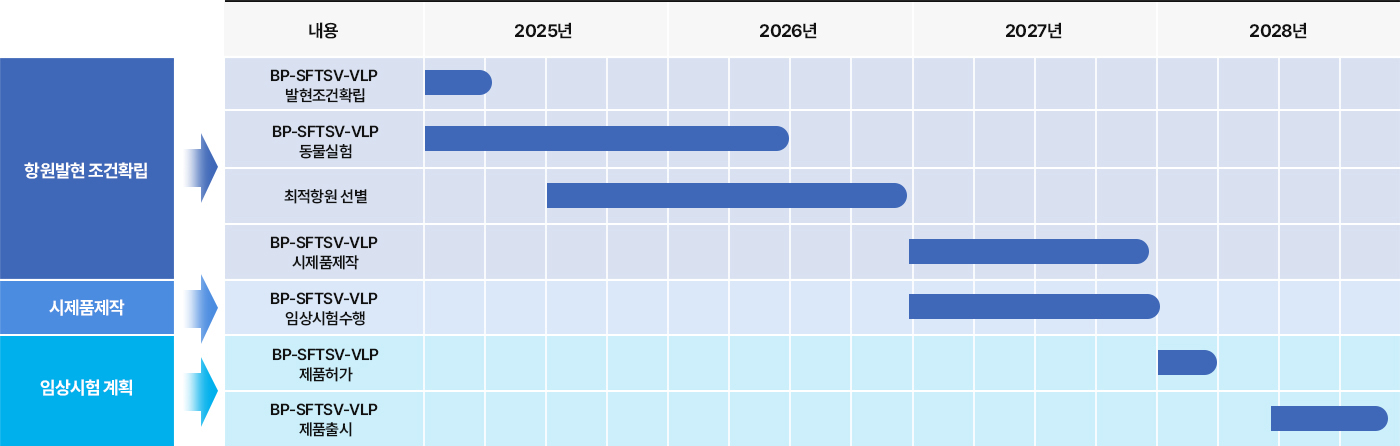
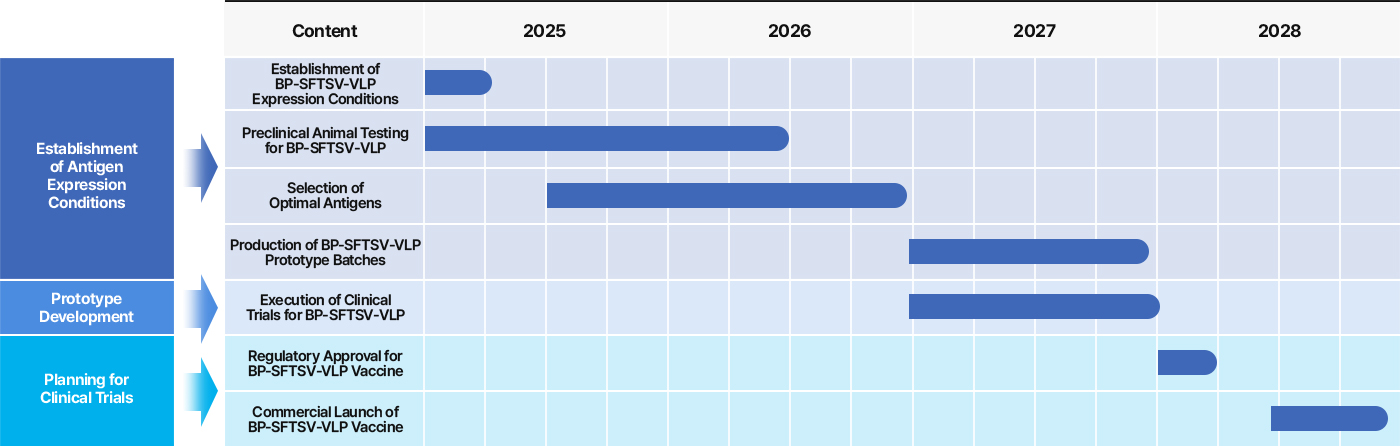
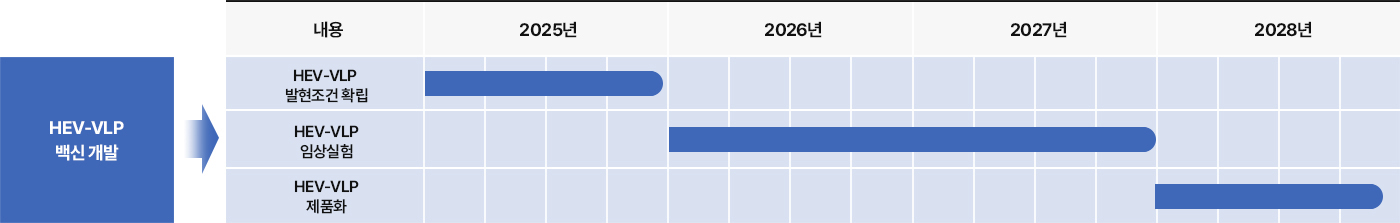
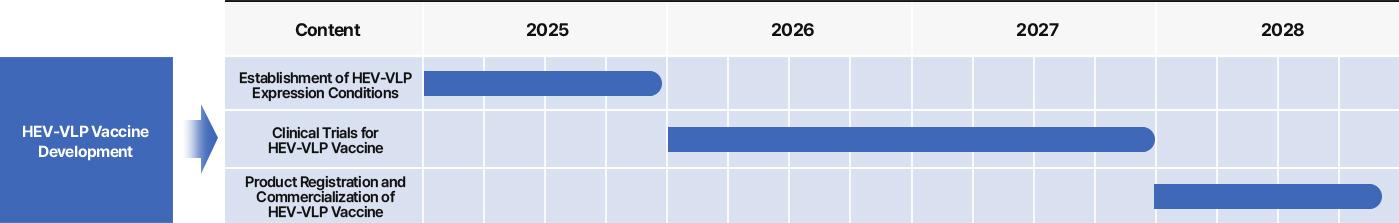
3 Nanovax’s HEV Vaccine
Nanovax Co., Ltd. is developing a VLP-based vaccine targeting HEV-3, utilizing the ORF2 gene derived from swine-origin Hepatitis E Virus genotype 3.
The vaccine is being produced using an E. coli–based VLP expression system, enabling an effective and cost-efficient solution for preventing HEV-3 infections.
-
-
1 What is SFTS (Severe Fever with Thrombocytopenia Syndrome)?
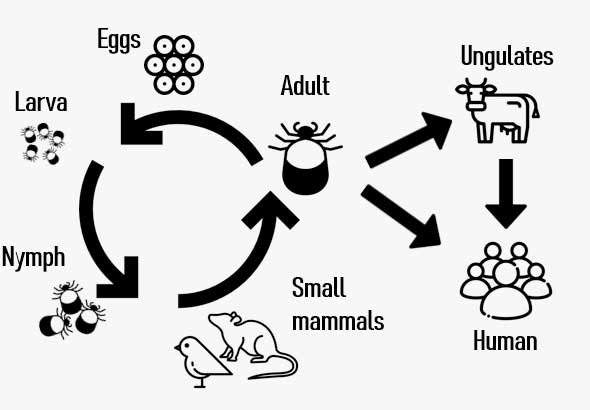
Severe Fever with Thrombocytopenia Syndrome (SFTS) is a fatal zoonotic disease transmitted by ticks and caused by infection with the SFTS virus (SFTSV).
In humans, SFTSV infection has a case fatality rate of 10–30%, with mortality exceeding 50% in vulnerable populations, such as the elderly and immunocompromised individuals, including young children

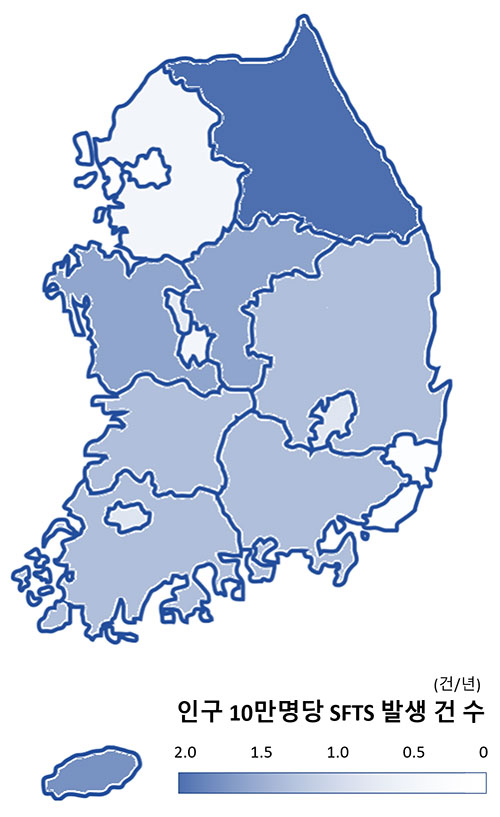
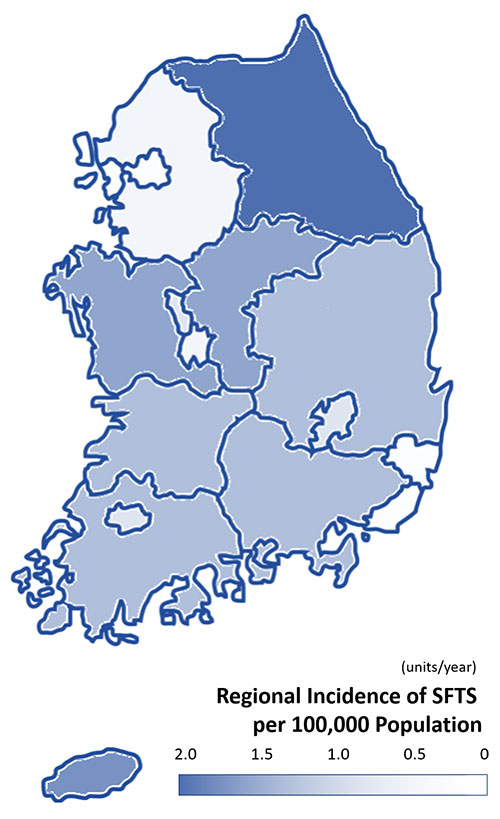
Regional Incidence of SFTS per 100,000 Population Seoul 0.05 Busan 0.15 Daegu 0.46 Incheon 0.07 Gwangju 0.41 Daejeon 0.21 Ulsan 0.18 Gyeonggi 0.16 Gangwon 1.82 Chungcheong 1.51 Jeolla 1.21 Gyeongsang 1.26 Jeju 1.63 Sejong 0.55 People infected with SFTS
Unit: NumberTicks → Humans Humans → Humans Dogs → Humans deceased 2013 36 17 2014 55 4 16 2015 79 5 21 2016 165 19 2017 272 2 54 2018 259 2 1 46 2019 223 41 2020 243 15 37 2021 172 26 2022 193 40 2023 198 38 2024 170 2 1 26 Total 2029 30 20 381 -
2 Problems due to absence of SFTSV vaccine
To date, there is no available vaccine against SFTSV (Severe Fever with Thrombocytopenia Syndrome Virus).
In South Korea, the geographic spread and number of reported cases continue to increase each year, largely due to climate change and the expansion of tick habitats.
As a result, the development of a safe and effective vaccine is urgently needed to prepare for and mitigate the growing threat of SFTSV.
-
3 Pioneering a New Paradigm in Infectious Disease Prevention Through Innovative Vaccine Technology!
Nanovax Co., Ltd. has successfully developed a BP-SFTSV subunit vaccine using recombinant protein technology, which demonstrated the ability to suppress SFTS-induced thrombocytopenia in mouse models.
This technology is expected to play a significant role in preventing the health impact of SFTS infections and offers promising potential for future vaccine development.
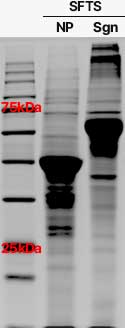
Confirmation of SFTS Subunit Expression
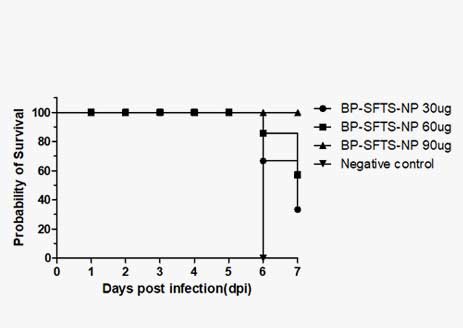
Analysis of Protective Efficacy Against SFTS
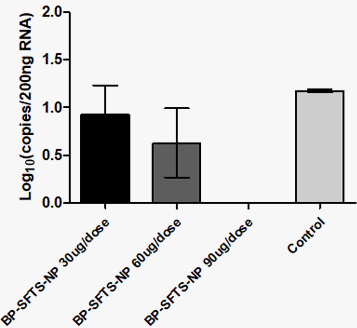
SFTSV 바이러스 여부 분석
-